India should enhance its regulatory framework to tackle emerging viral threats such as human metapneumovirus by strengthening surveillance, diagnosis, and rapid response mechanisms through National Health Mission initiatives. Investments in vaccine and antiviral research, along with global best practices, will help mitigate such outbreaks. Strengthening the healthcare infrastructure is essential to protect vulnerable populations and control HMPV spread.
Human metapneumovirus has emerged as a global health concern, particularly for vulnerable populations such as children, the elderly, and immunocompromised individuals. First identified in 2001, human metapneumovirus causes significant respiratory infections worldwide, leading to hospitalization and mortality, particularly in low-income countries. As viral outbreaks such as human metapneumovirus pose increasing challenges, it is important to strengthen regulatory frameworks for effective management. Human metapneumovirus has become a significant global health concern due to its widespread spread, particularly during seasonal outbreaks.
The virus is gaining attention as its impact increases across regions. In China, increased surveillance has revealed a rise in human metapneumovirus cases, particularly in children and the elderly, indicating an increase in detection rates. Although first identified in 2001, human metapneumovirus has gained attention worldwide due to its increasing respiratory infections, leading to increased hospitalizations and mortality. The World Health Organization reports that human metapneumovirus accounts for 3%–10% of hospitalizations globally, with a significant increase in children under five years of age.
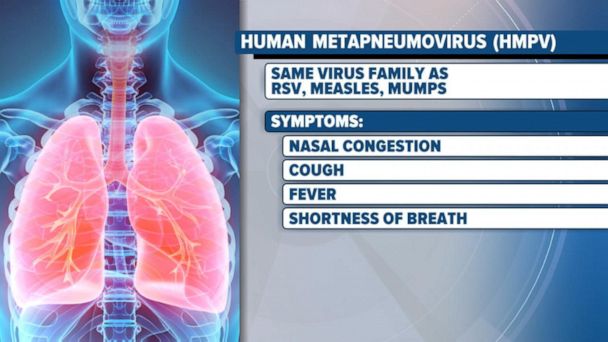
The virus often spreads rapidly during flu season, leading to a surge in respiratory infections, making it a seasonal health threat. In China, the flu season coincides with a surge in human metapneumovirus cases, leading to increased hospitalizations and media coverage. Despite being in circulation for years, many countries, including India, lack a comprehensive and affordable testing infrastructure for human metapneumovirus. While global agencies monitor human metapneumovirus, many regions still face challenges in detecting and reporting it, affecting early interventions. The virus disproportionately affects certain vulnerable groups, such as children under five years of age.
Human metapneumovirus disproportionately affects young children, particularly those under the age of five, who are at higher risk of severe illness and hospitalization. The elderly, particularly those with existing health problems, are more vulnerable to severe outcomes from human metapneumovirus infection. In China, a rise in human metapneumovirus cases in the elderly has led to a rise in hospitalizations, highlighting the vulnerability of the older population. People with weakened immune systems are at higher risk of severe infection with human metapneumovirus, requiring intensive medical care.
Deaths due to hMPV are significantly higher in vulnerable groups, including infants and immunocompromised people. The case fatality rate for children under five years of age is 1%, raising concerns about the impact of the virus on high-risk populations in both developed and developing countries. In low- and middle-income countries, limited healthcare access increases the risk of severe outcomes for vulnerable populations. Measures to strengthen the regulatory framework and capabilities to manage such viral outbreaks include strengthening the diagnostic infrastructure. India can enhance its diagnostic capabilities by expanding testing facilities for viruses such as hMPV in both the government and private sectors.
The Indian Council of Medical Research can collaborate with private laboratories to ensure widespread, affordable hMPV testing, leading to early detection and prevention. India can expedite the approval process for diagnostic tests by establishing a streamlined regulatory pathway that allows rapid approval of tests during public health emergencies. The Drugs Controller General of India can implement an emergency approval mechanism for diagnostic kits that meet international standards. A robust national surveillance system can be developed to monitor respiratory infections in real time, tracking trends and emerging threats such as human metapneumovirus.
The Ministry of Health and Family Welfare can expand the Integrated Disease Surveillance Programme to include real-time human metapneumovirus tracking across all states. India can launch public health campaigns to raise awareness about human metapneumovirus, focusing on prevention methods and recognizing symptoms, especially among vulnerable populations. The Ministry of Health can partner with the media and NGOs to conduct information campaigns, especially in rural areas, to recognize symptoms of respiratory infections such as human metapneumovirus.
Strengthen international collaboration: India can enhance its global collaboration to share information and resources for managing viral outbreaks, thereby ensuring timely response to emerging pathogens. India should enhance its regulatory framework to tackle emerging viral threats such as human metapneumovirus by strengthening surveillance, diagnosis, and rapid response mechanisms through National Health Mission initiatives. Investments in vaccine and antiviral research, along with global best practices, will help mitigate such outbreaks. Strengthening healthcare infrastructure is essential to protect vulnerable populations and control HMPV spread.






